CuiScine: Glutens

If ever there was a lament of the no-carbs-dieter, surely there would be no lament greater than the loss of that chewy, stretchy, spongy mouthfeel of bread. What is it that gives bread its signature texture, the one we crave in our most depraved food-binges, the one we share diplomatically at family meals?
While there are many factors contributing to the magic of bread, the one that is perhaps most pertinent to its texture is… gluten.
Yes, gluten! That villainous being, a pseudo-swear in modern society, seemingly responsible for all our health woes and, yes, that sinisterly seductive texture. However, gluten doesn’t deserve its bad rep. It’s just a little misunderstood.
Gluten is a protein found in wheat, barley, and rye. It is made mostly of two other proteins, glutenin and gliadin. The two make up about half each of the gluten structure. Gliadin is perhaps the more misunderstood member; it’s responsible for gluten sensitivity and Celiac disease. But before you throw rocks, it’s not all gliadin’s fault! Your body just has something against it.
When gliadin is consumed, it reacts with an enzyme in your body called transglutaminase. In some cases, the body decides this interaction is suspect, and causes an immune response with inflammation (think about when you get a rash). This is the root issue of Celiac disease, the most severe gluten-related disease. Some immune systems also respond specifically to gliadin itself, creating intestinal discomfort. This is what is known simply as “gluten-sensitivity”. So, although involved in harmful activities, it’s more of an innocent bystander that your body likes to bully.
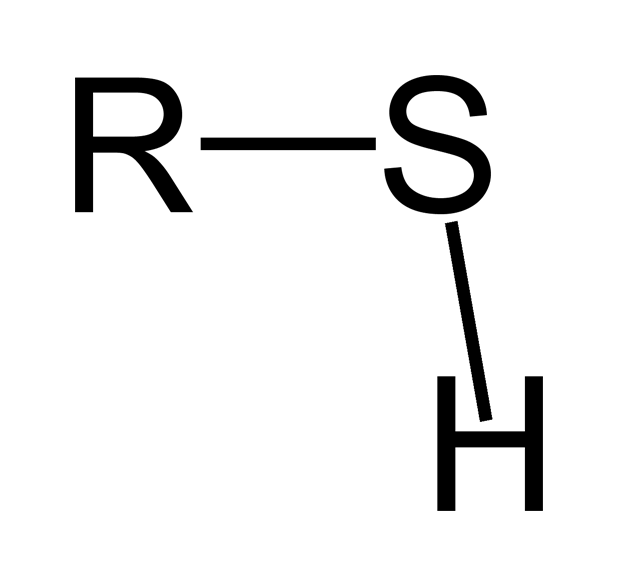
Gliadin is responsible for the viscosity of dough, and its more mobile qualities. Glutenin, on the other hand, is what gives dough its strength and elasticity. Glutenin molecules have a feature found in many places in nature called a “thiol” group- a hydrogen atom bonded to a sulfur atom on a larger molecule.

Sulfurs are conceited. They like to bond in pairs called “disulfide” bonds, but they’re weak and hard to achieve if they’re not close enough. Not only that, but hydrogen is in the way.
When dough is kneaded, the sulfur atoms from glutenins have more opportunities to get cozy because the molecules are moving more, increasing the chances of an encounter. When two sulfur atoms get close enough, an “oxidation” reaction occurs to rid them of those pesky hydrogens. Some manufacturers will even put oxidizers in their flour to facilitate the process! After the bond forms, the two gluten molecules (which are coiled in shape) are linked by their glutenins. This can happen hundreds of thousands of times to form enormous chains of coiled gluten molecules, which create that elastic quality. In fact, gluten molecules are among the largest proteins in nature.
After kneading, the next big change happens in the oven. Pliable dough goes in; stable, firm bread comes out. In the high heat, the gluten proteins will “denature” and lose their coiled shape. As they uncoil, they expose more of their sulfurs and bond alongside other gluten molecules. This bolsters the chains, making them less malleable and more firm. If you have ever wondered what makes bread (or cake, or any flour-based product) firm up in the oven, gluten is part of the equation.
The texture of bread and the breaking of bread are equally timeless. Splitting a loaf reveals that desirable toothsome nature- the nature of gluten. So don’t vilify this glorious molecule. You owe the best of bread to him.